New saliva technology makes big claims, but some experts still need convincing.
It may be some time before we have a simpler, non-invasive way to diagnose Sjogren’s disease, and it might not rely solely on saliva.
The diagnosis of Sjogren’s disease currently requires a minor salivary gland biopsy. While this is a fairly straightforward procedure, it is invasive and can have long-term consequences if complications arise. There is a clear need for a simpler, non-invasive method for predicting and diagnosing the condition.
Now, a new research study published in Arthritis Research & Therapy describes the identification and validation of a six-biomarker panel that could serve as a non-invasive tool for classifying Sjogren’s disease.
Chinese researchers collected saliva samples from 258 suspected patients, which were analysed using mass spectrometry and enzyme-linked immunosorbent assay. All patients also underwent the salivary gland biopsy to confirm the presence or absence of Sjogren’s disease.
The first step in the process was determining which salivary proteins differed between six patients who did have Sjogren’s and six patients who did not. Five immune-related proteins were found to be elevated in patients with the disease: complement C3, complement factor B, clusterin, calreticulin and neutrophil elastase.
Professor Maureen Rischmueller, a senior consultant with the rheumatology unit at the Queen Elizabeth Hospital in Adelaide, said that while it was important to identify a simpler test to diagnose Sjogren’s, the proposed saliva test was a long way off being translated into clinical practice.
“This sort of research is really interesting and it’s being done by a number of groups, but they all get different results. I look forward to [seeing] the evolution of these biomarker studies, [because] if they do find some that are replicable and useful, that will be fantastic,” she told Rheumatology Republic. “It’s just not there yet.”
Professor Rischmueller was concerned that this approach wasn’t any better than simply measuring the quantity of saliva someone produces, which is what is currently done in clinical practice.
“They’ve shown higher [protein] markers in Sjogren’s cases versus controls, but is it because they’re more concentrated in Sjogren’s? Saliva contains both mucus and water, and it’s the water component that’s reduced in Sjogren’s patients, which would clearly increase the protein concentrations,” she said.
Second, the researchers looked at these proteins in a larger sample (186 patients, 121 of whom had Sjogren’s) and confirmed the concentrations were higher in the cases compared to controls. Comparing these samples also revealed differences in 10 other clinical characteristics between groups.
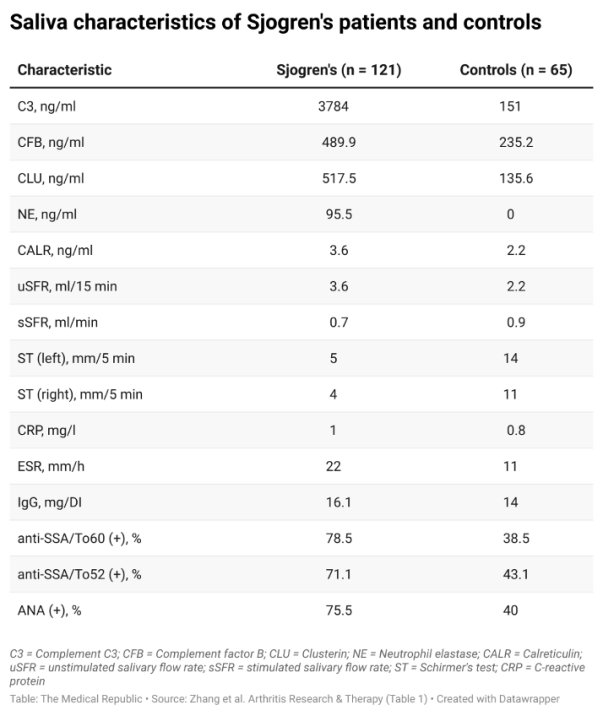
Finally, researchers tested whether the factors that differed in the saliva of Sjogren’s patients and controls could be used to discriminate the two groups. A series of regression models containing different combinations of the previously identified factors revealed that a six-marker model, containing complement factor B, clusterin, neutrophil elastase, Schirmer’s test and the presence of antibodies to Ro60 and Ro52, could identify Sjogren’s patients with 85% sensitivity and 92% specificity.
The predictive ability of the six-marker model was validated in a separate cohort of 72 patients, 49 of whom had Sjogren’s disease. The sensitivity and specificity in the validation set were 79% and 86%, respectively.
Related
The researchers felt that while the saliva-based test could still be improved by developing standardised saliva collection protocols, the test should be the first point of call when clinicians are presented with a potential Sjogren’s patient.
“As [the] biopsy is an invasive examination and the six-marker prediction model showed potential to replace [the biopsy], it’s recommended to use the model before [the biopsy in order] to reduce the number of unnecessary biopsies while missing fewer Sjogren’s patients,” they concluded.
A key limitation of the research was that the five immune-related proteins did not differ between patients with Sjogren’s, lupus and rheumatoid arthritis, which Professor Rischmueller said would a problem moving forward.
“Sjogren’s is often misdiagnosed as rheumatoid arthritis or lupus… [so] you would want a more specific test,” she said.
Professor Rischmueller explained that there had been significant progress with ultrasound techniques for imaging studies involving the salivary glands, which could potentially form part of a non-invasive diagnosis in the near future.
“The next iteration of the ACR/EULAR criteria will probably bring in ultrasound for the classification and diagnosis of Sjogren’s, because there was a paper a few years ago that show it’s got pretty good positive and negative predictability [compared] to the gold standard, which is salivary gland biopsy,” Professor Rischmueller told RR.
“That might be a non-invasive algorithm included in the [guidelines] so we don’t have to go on and biopsy so many people.”





