Historically, a clinical condition is first identified in its most advanced stage, when the manifestations are most obvious. Axial spondyloarthritis (axSpA) represents a spectrum of inflammatory conditions that primarily affect the axial skeleton and sacroiliac joints.1 Its most advanced state is ankylosing spondylitis (AS),2 a condition which was first recognised as a distinct entity at […]
Historically, a clinical condition is first identified in its most advanced stage, when the manifestations are most obvious. Axial spondyloarthritis (axSpA) represents a spectrum of inflammatory conditions that primarily affect the axial skeleton and sacroiliac joints.1 Its most advanced state is ankylosing spondylitis (AS),2 a condition which was first recognised as a distinct entity at the turn of the 20th century.1, 3
X-ray technology was developed around the same time but was not applied to the diagnosis of AS3 until the early 1920s. By the 1930s the use of radiography was able to show structural changes and establish that the disease normally starts in the sacroiliac joints.3
RADIOGRAPHIC EVIDENCE THE EARLY BENCHMARK
Based on this feature, radiographic sacroiliitis has become pivotal to the diagnosis of AS3 and was given an important role in the development of classification criteria, firstly in the early 1960s, and subsequently in 1984 with the establishment of the modified New York (mNY) criteria.1,4
The mNY criteria combined clinical and conventional radiographic findings and became the most widely used criteria until it was recognised that identifying sacroiliitis on X-ray was a late finding in the disease course of many patients.1
ESSG CAPTURES A WIDER DISEASE SPECTRUM
In the 1990s, the Amor and European Spondyloarthropathy Study Group (ESSG) criteria were created to capture a wider spectrum of spondyloarthropathies. However these criteria were too general to specifically identify early stage axial disease as they still required the presence of X-ray changes in the sacroiliac joints to establish a diagnosis.1,3
It is estimated that it takes on average a decade between onset of axSpA symptoms before these X-ray changes develop, although in some cases more rapid progression occurs.5
While the term “undifferentiated” spondyloarthritis (SpA) was frequently used to describe the disease using these criteria it could easily be misinterpreted as representing “not well defined SpA.”3
MRI BRINGS DISEASE PATHOLOGY TO LIGHT
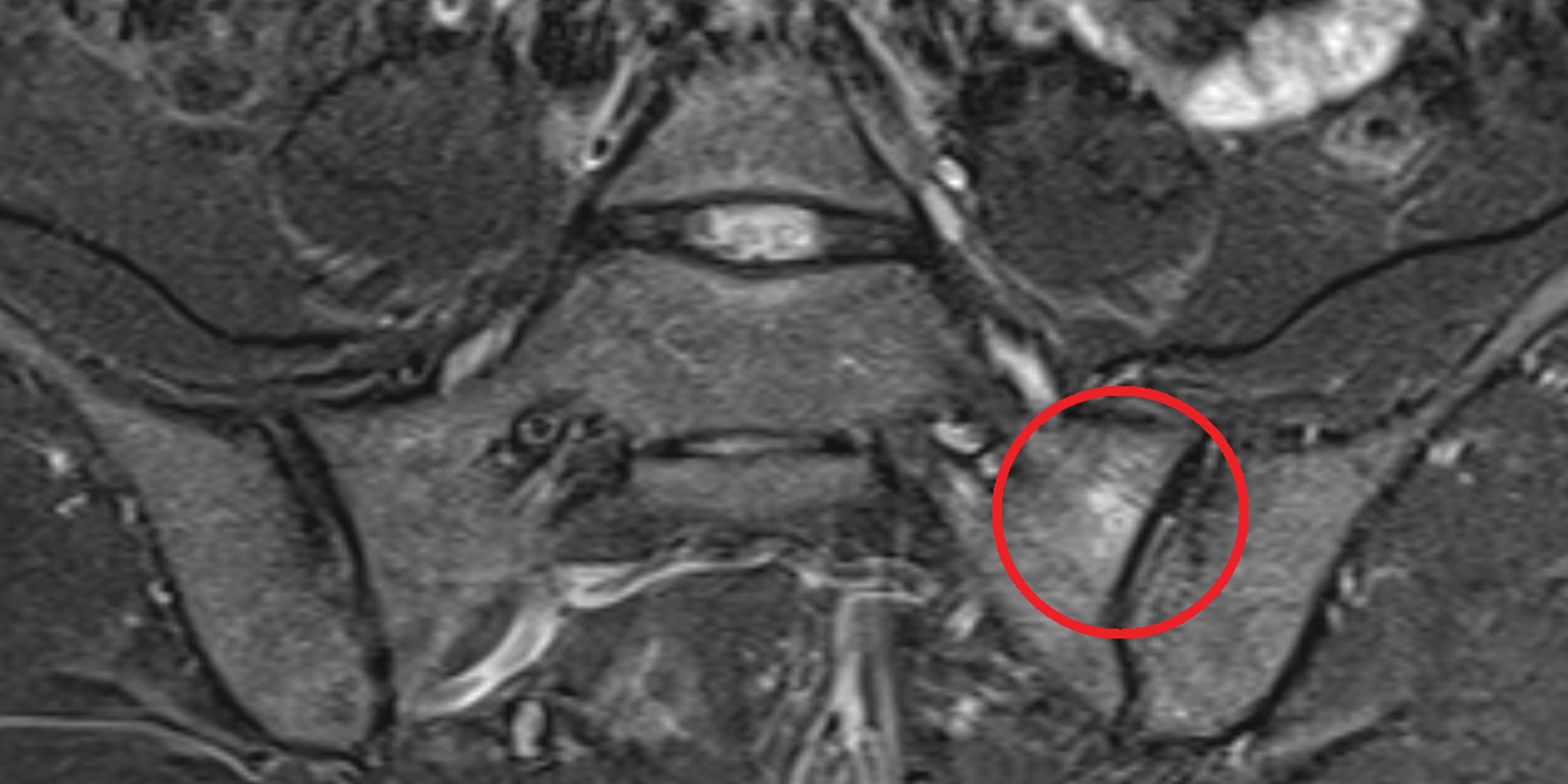
The advent of magnetic resonance imaging (MRI) enabled the visualisation of active inflammation at the sacroiliac joints and spine that is not evident on conventional radiographs.1
Active inflammatory lesions such as bone marrow oedema/osteitis, synovitis, enthesitis and capsulitis associated with SpA can be detected by MRI. Structural damage such as sclerosis, erosions, fat deposition and ankylosis can also be detected.4
MRI brought to light the long pre-radiographic phase that is universal in AS and led to the development of new classification criteria that do not require X-ray changes to be present.5
ASAS CRITERIA IDENTIFIES A NEW CLINICAL ENTITY – NR-AXSPA
The Assessment of Spondyloarthritis International Society (ASAS) criteria were established in 2009. They allowed an MRI finding of active inflammation of the sacroiliac joints as an alternative to radiographic sacroiliitis in the classification criteria of axSpA.1
The ASAS criteria allowed a clearer distinction between axial and peripheral SpA compared to the ESSG and Amor criteria.3 They resulted in the classification of a new clinical entity called non-radiographic axial spondyloarthritis (nr-AxSpA) which refers to patients with inflammatory axial arthritis without X-ray changes typical of AS.1 Moreover, these criteria were able to improve sensitivity and specificity compared to the ESSG or Amor criteria. They had a sensitivity of 82.9% and a specificity of 84.4%, compared to 70.7% and 63.5%, respectively, for the ESSG criteria and 69.4% and 78.4% for the Amor criteria.3
Nr-AxSpA and AS are now seen as distinct sub groups in the axSpA spectrum, occurring at consecutive stages, but with similar disease burdens and symptoms
DISEASE CONTINUUM
Nr-AxSpA and AS are considered now as two consecutive stages of one disease.7 Patients with nr-AxSpA may or may not eventually develop structural damage. However, radiographic sacroiliitis may take years to develop, if at all, complicating identification and delaying management of patients who may have earlier stages of the disease but do not present with evident signs of damage in the sacroiliac joints.8
Roughly half of patients presenting with nr-AxSpA will, over the space of a decade, progress to develop AS.5
It may slowly progress with visible radiographic damage showing little or minimal osteoproliferation. Or it may rapidly progress, causing damage and osteoproliferation with the formation of syndesmophytes and subsequent curvature of the spine. In some individuals, however, the disease may be arrested at an early stage without further progression and damage.2
PATHOLOGY OF PROGRESSION
Cohort studies have demonstrated that C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR], and especially a disease activity score incorporating CRP or ESR (ie, the Ankylosing Spondylitis Disease Activity Score [ASDAS]), were associated with radiographic spinal progression. However, there are limited data available to determine whether and which patients with
nr-AxSpA will develop structural changes in the sacroiliac joints and experience progression.Although the similarities and differences between nr-AxSpA and AS may suggest that for some patients, these classifications represent early and later stages of axSpA, it is not known whether this distinction is clinically relevant or warrants different treatment strategies.8
RATIO OF NR-AXSPA TO AS
Most of the available data for nr-AxSpA prevalence are from studies performed in patients with suspected axSpA, but without a clear diagnosis, who were then referred to rheumatologists. Nr-AxSpA or AS was then diagnosed for the first time. These studies indicate the proportion of nr-AxSpA patients among those with newly diagnosed axSpA ranges between 23% and 80%, depending on the symptom duration, selection criteria and other parameters, such as availability and interpretation of MRIs. Interestingly, some of the studies showed that radiographic sacroiliitis may already be present in 20-30% of patients after only two to three years of symptom duration.3
While there is a predominance of males among those with radiographic sacroiliitis, the proportion of men and women is equal or may even show female preponderance among those without radiographic sacroiliitis.2
SYMPTOMS
The inflammation and eventual progression of joint damage in some individuals with axSpA can cause significant impairment in physical function. Both AS and nr-AxSpA are frequently associated with both peripheral articular and extra-articular disease; these are equally prevalent in both groups, with the exception of uveitis. Uveitis appears to be more prevalent among patients with AS than nr-AxSpA, a difference that is not clearly related to differences in HLA-B27 status.1
Aside from uveitis, the clinical distinction between the two entities is not very pronounced, although nr-AxSpA patients are more likely to be female and less likely to be HLA-B27+.1
Several studies have shown that patients with AS and nr-AxSpA have similar levels of clinical disease as measured by the Bath Ankylosing Spondyloarthritis Disease Activity Index (BASDAI), functional status as measured by the Bath Ankylosing Spondyloarthritis Functional Index (BASFI), and health-related quality of life (HRQOL) measurements.1
DISEASE BURDEN
Real-world data from the US CORRONA registry showed that although patients with nr-AxSpA were younger and showed a trend for shorter symptom duration, they shared a similar disease burden to AS patients. This was reflected in comparisons of disease activity and function, quality of life, pain, fatigue, absenteeism, and work productivity loss (all p>0.05).8
A multi-perspective cross-sectional observation study in Europe found that clinical outcomes were consistently better in biological-treated versus -naïve axSpA patients. Of the patients who received treatment, responders had significantly less impaired activity than non-responders as measured by the Work Productivity and Activity Impairment questionnaire (33% vs. 47% respectively; p<0.001). Average pretreatment pain levels were 6.6 in biologic responders and 6.2 in biologic-naive patients (p=0.072) but reduced to 2.5 and 4.0 respectively (p<0.001) at the time of the survey. 13% of the nr-AxSpA patients in fact met the criteria for AS.9
Clinical trials have shown the gold standard of treatment for AS achieves similar responses in nr-AxSpA
CURRENT MANAGEMENT APPROACHES
As with the treatment of AS, management of nr-AxSpA is best performed by a multidisciplinary team. It is of particular importance to engage with radiologists to ensure they understand what is required for accurately reading MRI scans of inflammation of the sacroiliac joint. Multidisciplinary teams also include allied health professionals such as specialist rheumatology nurses, nurse practitioners and physiotherapists.5
BENEFITS OF BIOLOGIC TREATMENT
Clinical trials have confirmed that TNFi therapy, the gold standard treatment for established AS, is also effective in treating nr-AxSpA. This is particularly true for cases that are of more recent onset (< three years symptom duration), with elevated ESR or CRP levels, or a positive MRI scan providing objective evidence of inflammation.5 In such cases, the treatment response is similar to that seen with established AS, where TNFi treatment is highly effective.5
A long-term, two-year follow-up study with TNFi therapy demonstrated that early treatment may prevent radiographic damage and be associated with low disease activity or remission.10
In Australia, SIMPONI (golimumab) and etanercept are TNFis indicated for the treatment of nr-AxSpA in adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation as indicated by elevated CRP and/or MRI evidence, who have had an inadequate response to, or are intolerant to, non-steroidal anti-inflammatory drugs (NSAIDs).11,12 Recently the PBAC made a positive recommendation for SIMPONI to be included on the PBS for the treatment of nr-AxSpA.
SIMPONI has shown a rapid reduction in the signs and symptoms of nr-AxSpA and the effect was sustained over 16 weeks.13 Over 70% of patients achieved an ASAS20 response at the study end compared to 40% with placebo p<0.0001).13
The treatment group difference for the key secondary endpoint of ASAS40 was similar to the ASAS20 response (p<0.0001).13
Clinically meaningful improvements were also observed for key secondary measures including BASDAI 50 (p<0.0001), ASAS partial remission (p=0.0136) and SPARCC MRI SI score (p<0.0001).13
The data indicate that the treatment response in patients with nr-AxSpA was similar to the response seen in patients with AS.13


Editorial created by Janssen-Cilag Pty Ltd. Refer to the Product Information before prescribing, available from www.janssen.com.au/Simponi_PI
References:
1. Ghosh and Ruderman Arthritis Research & Therapy (2017) 19:286 DOI 10.1186/s13075-017-1493-8
2. Malaviya: Int J Rheumatol. 2017; 2017: 1824794. Published online 2017 May 7. DOI: 10.1155/2017/1824794
3. Sieper et al: Arthritis and Rheumatology Vol. 65, No. 3, March 2013, pp 543–551 DOI: 10.1002/art.37803
4. Akgol et al; Rheumatology 2014;53:497-501 DOI:10.1093/rheumatology/ket385 Advance Access publication 20 November 2013
5. Brown and Bradbury: https://www.mja.com.au/journal/2017/206/5/new-approaches-ankylosing-spondylitis
6. Slobodin MD: IMAJ, Vol 17, December 2015 picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-03025-3&d=201809061016933
7. Poddubnyy: //www.ncbi.nlm.nih.gov/pmc/articles/PMC3582305/
8. Mease et al: DOI: 10.1002/acr.23534
9. Sieper et al: Clinical and Experimental Rheumatology 2016; 34: 975-983.
10. Cantarini et al: //journals.www.com/md-journal/Fulltext/2015/07050/Effectiveness_of_Adalimumab_in_ Non-radigraphic.11.aspx
11. SIMPONI Product Information (28 November 2017). Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-03025-3
12. ETANERCEPT Product Information (9 January 2018). Available at: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05235-3&d=201811141016933
13. Sieper et al: Arthritis and Rheumatology Vol. 67, No. 10, October 2015, pp 2702–2712 DOI 10.1002/art.39257
14. Pharmaceutical Benefits Scheme (PBS). Available at: www.pbs.gov.au